Cancer Center at Illinois (CCIL) researcher Hua Wang and Ph.D. student Rimsha Bhatta
Cancer Center at Illinois (CCIL) member Hua Wang published new research with promising developments for the future of personalized cancer therapeutics. In a Nature Communications paper, the Wang lab – in collaboration with Illinois professors Shelly Zhang, an assistant professor in the Department of Civil & Environmental Engineering (CEE), Wael Mostafa, a clinical assistant professor in the Carle Illinois College of Medicine (CIMED), Qian Chen, an associate professor in the Department of Materials Science & Engineering (MatSE), and CCIL member Erik Nelson – reports a novel extracellular vesicle (EV)-based cancer vaccine.
Wang’s research focuses on the discovery of cancer immunotherapies, and these latest findings are a noteworthy milestone in that journey. “Compared to other immunotherapies we’re developing in our lab, EV-based immunotherapy is a type of personalized medicine with huge potential for translation to the clinic,” said Wang.
Watch the video at the end of this story to see Hua Wang and Rimsha Bhatta discuss the impact of their research.
EVs are nanosized vesicles secreted by nearly all types of cells including tumor cells. They are critical players in cell communication, carrying essential molecular signals to the interacting cells. Like a delivery truck, tumor-secreted EVs transport antigen mail to immune cell recipients with critical instructions for how to get to work. As such, EVs have been the focus of decades of immunotherapy research. However, there has been little success for cancer treatment applications.
That stands to change with a bold, new immunotherapy approach developed in the Wang lab that introduces a universal metabolic tagging technology to the EV, supercharging its capacity to upregulate the body’s immune system response. Standard practice for improving conventional vaccines is to include an adjuvant that will activate dendritic cells (DCs). However, in practice, simply mixing adjuvants with EVs often results in inefficient DC activation and low antitumor efficacy. This problem motivated Wang’s lab to develop a new approach that can integrate large quantities of the adjuvant directly onto the EV surface for increased modulation of DCs and elicitation of potent cytotoxic T lymphocyte (CTL) response.
“Scientists have been trying to exploit tumor EVs as a form of therapeutic cancer vaccine for years with the goal to improve the processing of EVs by DCs, the prominent type of antigen presenting cells in the body. The typical EV vaccine has not been effective to date,” said Wang. “That brings us to our project. We wanted to better integrate EVs with the adjuvant to amplify the CTL response and antitumor efficacy. One key challenge, though, was the lack of an effective strategy to functionalize EVs and bind sufficient adjuvants onto EVs. We now have a metabolic labeling technology that lets us do that, though. We found that when we label the cancer cell with the chemical tag, the EV carries the chemical tag and allows for the conjugation of a good number of adjuvant. These adjuvant-conjugated EVs can efficiently activate DCs during the cellular internalization process. The results are a superior DC activation effect, robust T-cell response, and antitumor efficacy against lymphoma, melanoma, and other types of cancer.”

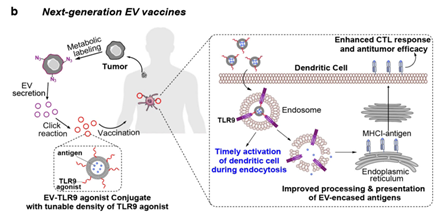
Wang’s lab uses a metabolic glycan labeling of parent cells, which can introduce chemical tags (e.g., azido groups) in the form of glycoproteins and glycolipids to the membrane of each cell, generating chemically tagged EVs (Figure 1a). They demonstrated that this EV-tagging approach is universally applicable to EVs secreted by various types of cancer cells, mesenchymal stem cells (MSCs), dendritic cells (DCs), and T cells.
This project not only marks a promising development in the big picture of personalized cancer immunotherapies, but it is deeply meaningful for Rimsha Bhatta, an Illinois doctoral student who has worked for years studying EVs. “This project has been very close to my heart. I’ve worked on EVs since my first year as a Ph.D. student in Prof. Wang’s lab. When I learned about this important area of cancer research, I wanted to work on developing a vaccine for human application. The Cancer Center at Illinois and the Materials Science and Engineering department allowed me to combine my interest in EVs and vaccines and bring a solution to the table for cancer patients. Our results have been very encouraging, and I am hopeful for the future of this project,” said Bhatta.
This foundational research into an EV-based cancer vaccine demonstrates the critical role of the CCIL, which provided the seed funding to help Wang generate an initial proof of concept, enabling the team to move forward on the research project. “The CCIL is a natural home for our lab and has been critical for our research. I appreciate the opportunity with the CCIL to build a growing collaboration network,” said Wang.
“Hua’s idea to develop an EV-based vaccine was very risky. Historical efforts to vaccinate against cancer haven’t proven successful. So, his bold research proposal required investment by the CCIL to generate proof of concept. Hua’s team took that initial data with support from the CCIL and acquired federal funding; it was a high-risk proposal. But the results are promising. This will be the first publication among many, no doubt,” said CCIL Cancer Discovery Platform (CDP) Program Leader Erik Nelson, who collaborated on this project.
This project presents a beautiful portrait of interdisciplinary collaboration possible on the Illinois campus. Wang’s lab pulled in the strengths of Prof. Nelson from the Department of Molecular and Integrative Physiology, Prof. Mostafa from CIMED, Prof. Zhang from CEE, and Prof. Qian Chen from MatSE. “I am happy to be a part of such a diverse team developing a clinically translatable therapeutic cancer vaccine,” said Zhang. “This fruitful collaboration at Illinois benefits from our current DARPA project on developing programmable biomaterials for biomedical applications. I look forward to working together to further explore how new programmable materials can impact cancer research.” Chen added, “This work also builds the foundation for our collaboration work with Prof. Wang on our current AFOSR project, to utilize advanced electron microscopy to understand the working mechanism of this metabolic tagging approach.”
What’s next for this research team? Moving forward, Wang said that his lab plans to collaborate with Prof. Mustafa to obtain patient-derived glioblastoma tissue, culture the samples in the lab, generate chemically tagged EVs, test in humanized-mouse models, and hopefully move toward human clinical trials.
Editor’s notes:
Hua Wang is a Cancer Center at Illinois (CCIL) member, a professor of materials science and engineering, and is affiliated with the Departments of Materials Science and Engineering, Bioengineering, the Beckman Institute, and the Carl R. Woese Institute for Genomic Biology.
To contact Hua Wang, email huawang3@illinois.edu
This research was supported by funding from the National Science Foundation (NSF), the National Institutes of Health (NIH), the U.S. Defense Advanced Research Projects Agency (DARPA), the Air Force Office of Scientific Research, the Department of Materials Science and Engineering at the University of Illinois at Urbana-Champaign, and the Cancer Center at Illinois (CCIL).
The paper “Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines” is available online.
https://doi.org/10.1038/s41467-023-43914-8
Written by Jonathan King, CCIL Communications Specialist