Urbana, Ill. – Estrogen receptors, proteins that promote cell growth, are therapeutic targets for endocrine-based breast cancer therapies. These therapies, including aromatase inhibitor treatments, perform well against breast cancer cells with normal, “wild type” estrogen receptors by blocking the formation of estradiol, the endogenous ligand that activates the receptor. However, several mutations in the estrogen receptor can develop over time that allow this receptor to work without the aid of a ligand, and so breast cancers with these mutant receptors become resistant, no longer responding to aromatase inhibitor treatment.
Cancer Center at Illinois (CCIL) scientists John Katzenellenbogen (professor of chemistry), Emad Tajkhorshid (professor of biochemistry), and Christopher Mayne (former graduate and postdoctoral research associate) recently published their findings on these mutations and their effects on estrogen receptor structural conformations in Molecular Cancer Research.
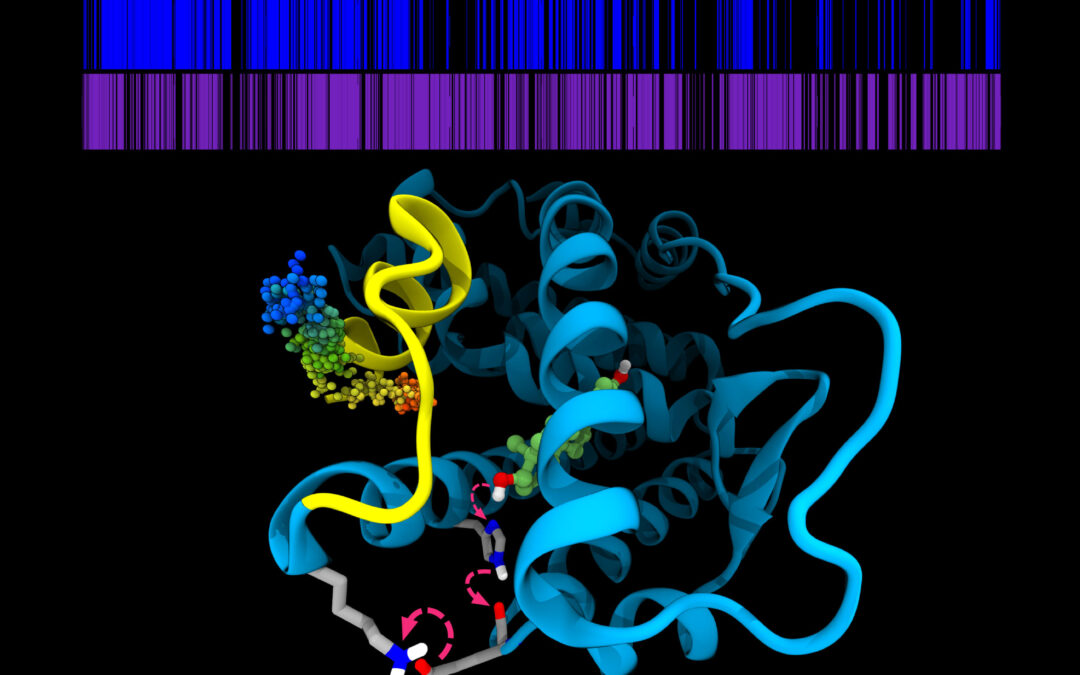
The group’s research began eight years ago, inspired by previous discoveries of mutations that allow estrogen receptors to activate without the presence of a ligand. Whereas the normal state of the receptor is unstable and unstructured, these mutations stabilize the active conformation, allowing breast cancer cells to grow unchecked.
“Imagine a spring that is also a switch, and you have to bend the spring to touch the ends together to make the switch work. The ligand works to make the ends stick together,” Katzenellenbogen said. “One mutation we found makes the spring longer and weaker so that this bending is easier, and the other introduces a kink in the spring so it is already bent in the first place.”

John Katzenellenbogen
However, in the early stages of their research, simple simulations and the technology available at the time could not explain what was happening in these mutations.

Christopher Mayne
Over time, Mayne developed his knowledge in molecular modeling and x-ray crystal structures as a student in Katzenellenbogen’s lab and continued to hone his computational skills in Tajkhorshid’s National Institutes of Health (NIH) Biotechnology Center for Macromolecular Modeling and Bioinformatics as a postdoctoral associate. Eventually, with the impressive resources and supercomputers offered by Tajkhorshid’s NIH center, Mayne was able to analyze the structural dynamics of the estrogen receptor mutations in extreme detail.
“We knew for a while that the mutations had something to do with the receptor’s movement, but we were unsure how,” Mayne said. “It was particularly challenging because of a lot of difficult-to-understand particularities, and we had to leverage cutting-edge technology to run our simulations and assays.”
“The synergy between computation and biomedical sciences, different disciplines and labs, was really powerful. Something was missing at the molecular level of explanation — how the structure moves and how the parts affect each other. We wanted to answer why and how this receptor can be activated without the presence of a ligand,” Tajkhorshid said.
Mayne’s models led to a prediction founded on the energetic basis of these conformational states. Prior to this research, there was no measure of the energetic activity and stability of the estrogen receptors in breast cancer cells with wild type and mutant receptors. Mayne investigated the “on” — or activated — and “off” states, but more importantly, the energy barrier between them.
“Research doesn’t happen in huge jumps. We slowly teased out what was happening, but it took conviction and a willingness to take the time to tie all the steps together,” Mayne said. “I don’t think this could have been done anywhere else in the world. We needed the experts at Illinois that understood all of the different aspects of this problem, who were willing to go out on a limb and test these theories.”
Emad Tajkhorshid
The researchers are optimistic that their findings may lead to more answers that will help scientists and clinicians better understand these molecules and mechanisms. Further studies may include more in-depth examinations of the hydrogen-bonding networks that seemingly play an important role in the switch from “on” to “off” states.
“The link between better understanding and improved health is not always a direct one. The problem is that the treatment affects what mutations you will get, and advantageous mutations that favor the opposite of what the therapy is trying to do will occur,” Katzenellenbogen said. “But if you take a deeper look, at an energetic level, at mutations that drive resistance, then we can better understand how to improve and provide guidance for drug development.”
– Written by the CCIL Communications Team
John Katzenellenbogen is a Research Professor of Chemistry at the University of Illinois and a researcher at the Cancer Center at Illinois.
Emad Tajkhorshid is a J. Woodland Hastings Endowed Chair in Biochemistry, Director of the NIH Biotechnology Center for Macromolecular Modeling and Bioinformatics, and a researcher at the Cancer Center at Illinois.
Christopher Mayne is a former University of Illinois PhD student in the Katzenellenbogen lab and postdoctoral fellow and staff scientist in the Tajkhorshid lab. Mayne is now affiliated with Loxo Oncology @ Lilly, Boulder, CO.