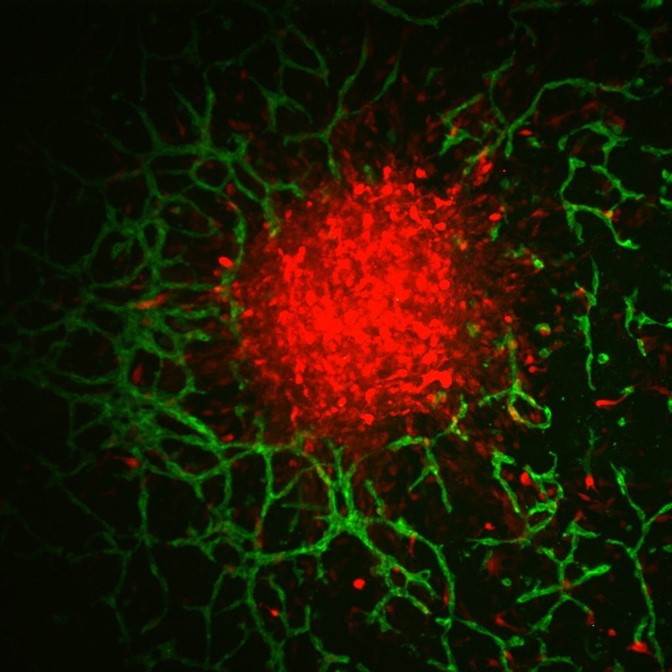
Glioblastoma spheroid vasculature.
Urbana, Ill. – Glioblastoma is the most common and lethal form of primary brain cancer. One of the reasons it is so hard to treat is that it spreads rapidly and diffusely throughout the brain, using structural features such as blood vessels to guide this invasion. Directly next to blood vessels exists a space called the perivascular niche comprised of several types of resident cells. In the brain, these include endothelial cells, astrocytes, and pericytes, which may impact glioblastoma formation and behavior.
Traditional two-dimensional culture models are limited in characterizing the relationship between the perivascular niche (PVN) and brain cancer cells, as they are not indicative of a brain tissue environment and do not provide a vascular component to study. Studies have shown that small clusters of glioblastoma (GBM) cells reside within the PVN, presenting an opportunity to investigate the formation and progression of the disease.
Mai T. Ngo, first author and former PhD student in Cancer Center at Illinois research program leader Brendan Harley’s lab, proposed the use of a 3D hydrogel tissue model to observe the interactions between tumor cells and brain vasculature, and to test potential therapeutic regimens for GBM patient treatment. Harley supervised the project, and Jann N. Sarkaria, a Mayo Clinic collaborator, provided patient-derived cell lines for the study.

Pictured Left to Right: Brendan A. Harley and Mai T. Ngo
The group’s main interest was to characterize the signaling between vascular and tumor cells, and to determine whether those signals impacted GBM growth. Other researchers have studied endothelial cells at length, but the roles of pericytes and astrocytes, cells that support and wrap around brain blood vessels, had yet to be elucidated.
“We wanted to create a next-generation tissue model that incorporated vascular cells and primary tumor cells from patients to make a brain-specific platform to study patient-specific responses,” Ngo said.
The gelatin-based hydrogel model was developed in Harley’s lab and has been shown to support the growth of GBM cells and allow the observation of tumor behaviors such as proliferation in a realistic brain tissue environment. For Ngo’s study, it also provided the ideal platform to grow brain vasculature alongside tumor cells.
“The exciting potential for this work is that it shows a route to create tissue engineering models to study how the brain microenvironment shapes spatial and temporal patterns of GBM cell invasion and drug response. These are difficult to study in a patient,” Harley said.
Finding an effective therapeutic regimen for GBM patients can be time-consuming and costly. Ngo’s study, which also tested the therapeutic response to chemotherapy in the artificial brain environment, had much shorter testing times with a maximum duration of nine days. The method is also more material effective, requiring thousands of cells for a test compared to the hundreds of thousands to millions needed for traditional testing in animals.
“With our model, you can test the response and determine the best treatment for a patient within a much shorter period of time. And it’s a ‘plug-and-play’ model, suggesting the opportunity to develop perivascular models of other tissues such as lung or breast,” Ngo said.
Written by Cancer Center at Illinois Communications.
Mai T. Ngo is now a postdoctoral researcher at Boston University, where she continues to study blood vessels for therapeutic applications.
Brendan Harley is a Cancer Center at Illinois research program leader, Robert W. Schaefer Professor in Chemical and Biomolecular Engineering, and research theme leader in the Carl R. Woese Institute for Genomic Biology at the University of Illinois Urbana-Champaign. Harley’s lab mainly pursues the fabrication, characterization, and testing of biomaterials for in vivo and in vitro tissue engineering applications.
The paper, “Perivascular Stromal Cells Instruct Glioblastoma Invasion, Proliferation, and Therapeutic Response within an Engineered Brain Perivascular Niche Model,” is available online. DOI: 10.1002/advs.202201888