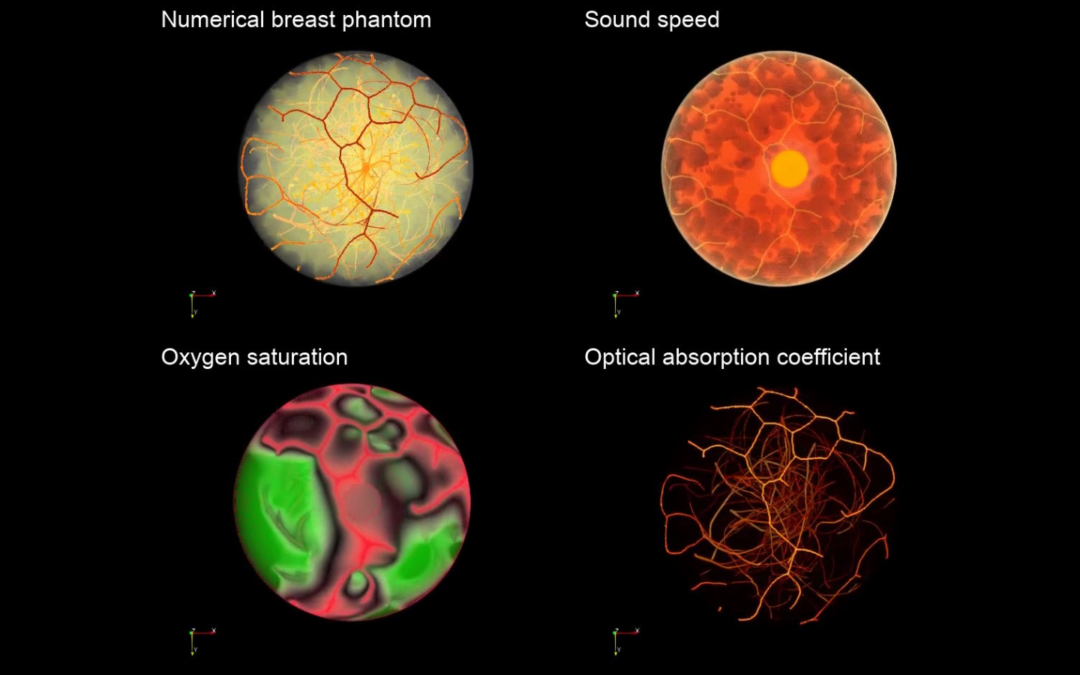
Image of numerical breast phantoms.
Urbana, Ill. – When new medical imaging systems are designed, they must undergo robust assessments and refinements to ensure that the images they produce contain as much diagnostically-relevant information as possible. Until now, this meant building machines from scratch, testing them, taking them apart, and reconfiguring them.
Photoacoustic computed tomography (PACT), also known as optoacoustic tomography, is an up-and-coming hybrid-physics imaging modality that synergistically combines the rich contrast of optical imaging with the high spatial resolution of ultrasound. Because it can provide functional information based on tissue oxygenation, PACT holds great potential for the assessment and management of breast cancers that are characterized by low oxygen environments due to atypically high metabolic activity in tumor tissues.
As an emerging medical imaging technology, PACT, currently lacks a practical validation or optimization technique to test and inform the design of systems. A collaborative research team led by Cancer Center at Illinois researcher, Mark A. Anastasio, seeks to develop computational methods to solve this problem for PACT and other novel imaging systems.
“It is generally impractical to conduct clinical trials to systematically refine new technologies such as PACT in their early stages of development due to financial and ethical concerns. As such, there remains an important need to develop methods for refining and optimizing new medical imaging technologies that can accelerate their development towards eventual clinical use,” Anastasio said.
Anastasio, head and professor of bioengineering at the University of Illinois Urbana-Champaign (UIUC), and collaborators were recently awarded $2.3M from the National Institutes of Health (NIH) for this project.

Mark A. Anastasio
“The broad objective is to develop a computational framework for simulating different PACT imaging system designs in a practical way. Groups that are developing PACT breast imaging systems generally adopt different system designs and no one knows which is the most effective,” Anastasio said. “So, we aim to provide a capacity that will enable the investigation of an imaging system design in silico and optimize it by use of clinically relevant metrics. In this way, promising system designs can be identified and compared before a physical imaging system is even constructed.”
The team’s specific aims are to develop, validate, demonstrate, and refine computational tools for performing virtual imaging trials (VITs) capable of assessing new imaging concepts and technologies such as PACT.
Traditionally, when research groups or companies seek to develop new imaging systems, they must physically construct the system. With trial and error, there can be much time and funds wasted on optimizing these systems. VITs represent a new paradigm in the field of biomedical imaging, allowing researchers to use computer simulations to model, assess, and monitor new devices.
“Each group has been ad hoc piecing together their imaging system without the ability to do comprehensive and systematic virtual assessments that would reveal opportunities for improvement. With our proposed computational methods, researchers will be able to design better systems and bring groundbreaking technologies to the clinic, and sooner,” Anastasio said.
Video of numerical breast phantoms.
A critical component of the VITs is developing digital 3D phantoms of the female breast, as they convey realistic anatomical structures and physiology. The researchers can virtually image these phantoms and produce measurements, which would normally be measured in experiments, and determine optimal designs of imaging systems and image formation algorithms.
Anastasio anticipates that this technology will be beneficial for cancer imaging, as it exploits characteristics of tumor tissue including metabolic and oxygen status of hemoglobin, angiogenesis, and other biomarkers. He also foresees potential opportunities to leverage this tool to aid diagnostics and monitoring of treatment response for other cancers, including brain and skin cancers.
Written by CCIL Communications.
Mark A. Anastasio is head and professor of bioengineering at the University of Illinois Urbana-Champaign. His lab addresses the engineering and scientific principles of biomedical imaging and Anastasio’s current research projects include advanced image reconstruction methods for breast cancers, which also requires high-performance computing methods.
Collaborators on this project include Frank Brooks (UIUC bioengineering research faculty), Seonyeong Park (postdoctoral researcher, UIUC), Umberto Villa (research scientist, University of Texas at Austin), Mohammad Eghtedari (radiologist, University of California San Diego), and Andre Kajdacsy-ball (pathologist, University of Illinois Chicago).